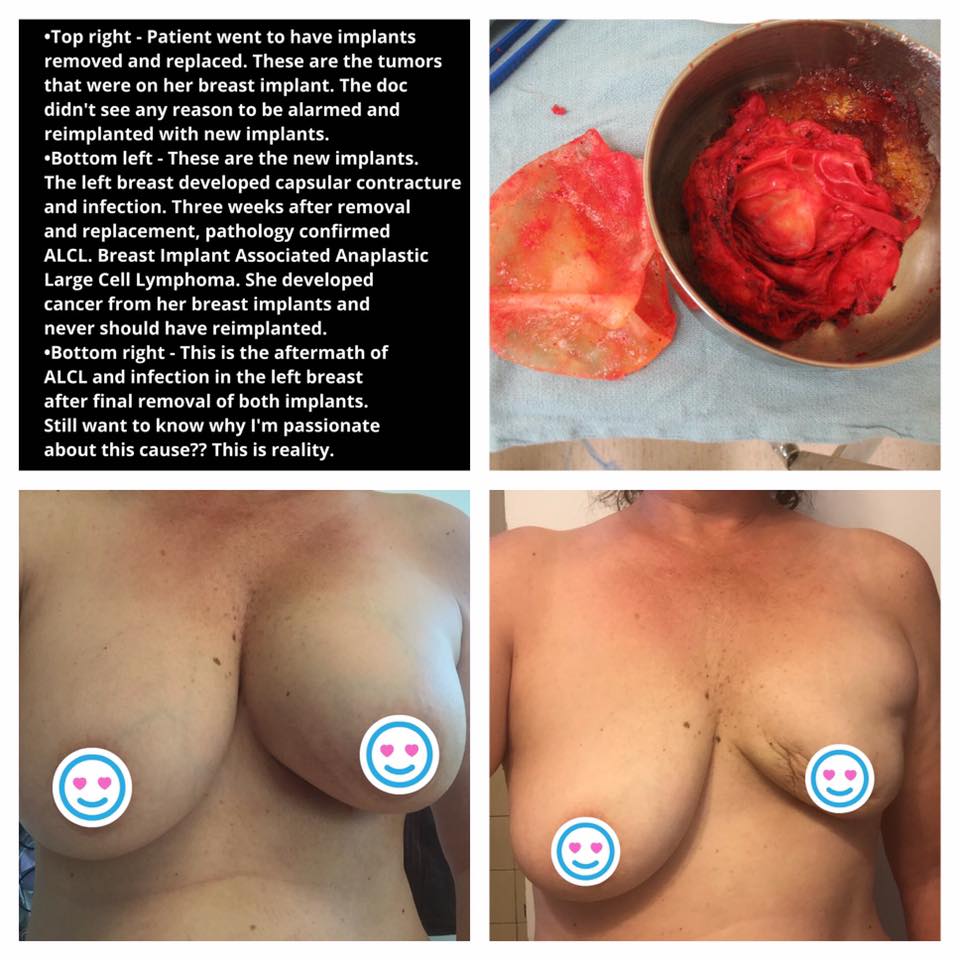
Symptomatic? Having trouble getting answers? What documents can I take to my doctor to educate them on BIA-ALCL?
Symptomatic? Having trouble getting answers? What documents can I take to my doctor to educate them on BIA-ALCL?
While the following information is not all-inclusive, it is good information to have, it is straightforward and fairly quick to read.
Clinical Information
-
National Library of Medicine (May 18, 2022)
- National Library of Medicine (April 25, 2022)
- Government of Canada (April 6, 2022)
- National Library of Medicine (March 24, 2022)
- National Library of Medicine (March 2, 2022)
- American Society of Plastic Surgeons – (Updated February 2020)
- National Comprehensive Cancer Network – (Updated January 2020)
- Finding Concensus after two decades of BIA Anaplastic Large Cell Lymphoma– (October 28, 2019)
- Risk of BIA-ALCL in a Cohort of 3546 Women Prospectively Followed After Receiving Textured Breast Implants– (June 12, 2019)
- Dr. Mark Clemens presentation during the FDA meeting about BIA-ALCL– YouTube Video(March 25, 2019)
- Dr. Mark Clemens FDA Advisory Panel Presentation – PDF file (March 25, 2019)
- Stepwise EnBloc Resection of Breast Implant-Associated Anaplastic Large Cell Lymphoma with Oncologic Considerations (February 27, 2019)
- CD30 Elisa – Research in progress for easier, cheaper test (February 21, 2019)
- National Comprehensive Cancer Network The NCCN guidelines for diagnosing and treating BIA-ALCL are available for free at nccn.org. The essential elements of these guidelines are summarized in two of the articles below. These guidelines are probably the most important documents to give your doctor.
- Aesthetic Society News (Winter 2018) Pages 6, 7 and 9.
- Joint ASPS / ASAPS Statement
- ALCL Brochure Tri-Fold
- Diagnosis and treatment of BIA-ALCL (The essential elements of the NCCN guidelines are summarized in Figure 1)
- How I Treat BIA-ALCL (The essential elements of the NCCN guidelines are summarized in Figure 4)
- BIA-ALCL: a good practice guide, pictorial review, and new perspectives
- An Updated Approach and Understanding of BIA-ALCL
- Primary Lymphomas of the Breast: A Review
- Current Progress in BIA-ALCL
FDA Announcements
- Allergan Recalls Natrelle Biocell Textured Brest Implants Due to Risk of BIA-ALCL Cancer (The FDA identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death. (September 12, 2019)
- FDA takes action to protect patients from risk of certain textured breast implants; requests Allergan voluntarily recall certain breast implants and tissue expanders from market (July 24, 2019)
- Statement from FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D., and Jeff Shuren, M.D., J.D., director of the FDA’s Center for Devices and Radiological Health on FDA’s new efforts to protect women’s health and help to ensure the safety of breast implants (May 2, 2019)
- Letter to Health Care Providers (February 6, 2019)
- Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) (August 2018)
- Anaplastic Large Cell Lymphoma (ALCL) In Women with Breast Implants: Preliminary FDA Findings and Analyses (January 2011)
Videos
- Talking with your Patients about BIA-ALCL (December 18, 2019)
- THE PLASTIC SURGERY CHANNEL: Maximizing Breast Implant Patient Safety: Raising Awareness of BIA-ALCL Part 3 of 3 (November 14, 2018)
- THE PLASTIC SURGERY CHANNEL: Maximizing Breast Implant Patient Safety: Raising Awareness of BIA-ALCL Part 2 of 3 (October 29, 2018)
- THE PLASTIC SURGERY CHANNEL: Maximizing Breast Implant Patient Safety: Raising Awareness of BIA-ALCL Part 1 of 3 (October 22, 2018)
- Video Mark Clemens ALCL an 2018 Update (September 24, 2018)
- Dr. Eric Swanson, BIA-ALCL & Textured Implant Use (September 9, 2018)